Is the Least Soluble in Water
Because it is non polar and non polar substances are insoluble in water. Which compound would be least soluble in water.
Solubility Factors When Choosing A Solvent News Announcements Cayman Chemical
When the two are mixed together the CCl4 sinks to below the water giving two layers of liquid.

. At 30C 90g of sodium nitrate is dissolved in. 15 Is salt water a polar solvent. Solve any question of Equilibrium with-.
Solubility Curves - Basic Introduction - Chemistry Problems Solubility Curves - Saturated Unsaturated Supersaturated Solutions Reading solubility curves Types of Solutions and Solubility Curves Solubility Curves Of Salts Experiment. How many grams of potassium chloride can be dissolved in 200g of water at 80C. T under the one which would be soluble in water.
Check Answer and Solution for above Chemistry q. As examples ethanoic acid is soluble in water. If N a ion and S2 ion is larger than Cl ion which of the following will be least soluble in water.
Hexane CH3CH2CH2CH2CH2CH3 is a hydrocarbon and nonpolar. Solution For Which is least soluble in water MNR 1984 89 Solution For Which is least soluble in water MNR 1984 89 Solution For Which is least soluble in water MNR 1984 89 About Us Become a Tutor Blog Download App. Which salt is least soluble in water at 20C.
The d-and f-Block Elements. 9 When salt is dissolved in water water is the. Which is least soluble in water A Na2S B MgS C MgCl2 D NaCI.
Both ammonia nh3 and phosphine ph3 are soluble in water. Writeeast under the one which would be least soluble in water. 12 Why sodium chloride is more soluble in water.
Solubility curves which salt is least soluble in water at 20 c solution crossword answer key below. It is a less ionic so that least soluble in water. But when those compounds molecular mass increases solubility in water is decreased.
Comments to the instructor. According to table f which compound is soluble in water Which compound becomes less soluble in water as the temperature of the solution is increased Which of the following alkaline earth metal sulphate is least soluble in water. Therefore AgF will be the most ionic compound and so it will be most soluble in water while AgI will be the least soluble.
It was observed that A started boiling after B when both were subjected to same conditions. 0At 40C how much potassium nitrate can be dissolved in 300g of water. 10 Why do things dissolve in water.
The halide of alkali metal least soluble in water. Which salt shows the least change in solubility from 0- 100C. Hence option A is correct.
Arrange the following compounds in order of increasing solubility in water. 13 Is salt water more polar than water. The solubility of carbon tetrachloride in water at 25 C is 0081 g100 ml water and is otherwise immiscible with water having a density of about 16 gml.
O2 LiCl Br2 CH3OH Like dissolves like. Both of compounds are carboxylic acids. However non-miscible liquids do display very low mutual solubility.
The halide of alkali metal least soluble in water. But benzoic acid is not soluble in water. Butanoic acid heptanoic acid formic acid Write lowest under the compound with the lowest boiling point.
11 Why does sugar and salt dissolve in water. Alcohols carboxylic acids carboxylic acid chlorides amines esters are usually soluble in water. Which of the following is the least soluble in water.
That is polar compounds usually are soluble in water and non-polar compounds are not soluble in water. Read more on solubility here. Based on Table F the chemical compound which is least soluble in water is because phosphates are generally insoluble.
Correct option is D Bigger is the size of anion higher is the covalent character and lesser is the solubility in polar solvents like water. A salt is soluble if it dissolves in water to give a solution with a concentration of at least 01 moles per liter at room temperature. Which of the following silver halide is insoluble in water but soluble in liquid ammonia.
Which is least soluble and why. Which is least soluble in water. Two compounds A and B were being tested for their boiling points.
In Chemistry a chemical substance or compound is said to be soluble if it dissolves completely in a solvent such as water. So AgI is least soluble of the given options. Propanal has a slightly higher boiling point than propanone.
16 What makes a salt soluble or insoluble. A salt is insoluble if the concentration of an aqueous solution is less than 0001 M at room temperature. Covalent compounds are insoluble in water.
17 What makes. 3 rows The gas which is most soluble in water is NH3 because of hydrogen bonding with water. 14 How does salt affect water.
Using the axiom like dissolves like hexane would water-insoluble.

Is Ch3oh Methanol Soluble Or Insoluble In Water Youtube

Vitamin C Autoimmune Skin Calm Inflammation Skin Care Gifts

Both Ammonia Nh3 And Phosphine Ph3 Are Soluble In Water Which Is Least Soluble And Why Lisbdnet Com

Solubility Introduction To Chemistry
1 6 Physical Properties Of Organic Compounds Chemistry Libretexts

Pin By Alyssa Jackson On Chemistry Education Chemistry Education Reactions Geeky

Water Soluble Colored Pencils 8 Articles Carrie L Lewis Artist Color Pencil Drawing Colored Pencils Watercolor Pencil Art

Dr Aid Factory Price High Quality Water Soluble Npk Fertilizer 20 20 20 Te Fertilizer Potassium Nitrate Seed Germination

Soluble And Insoluble Compounds Chart Solubility Rules Table List Of Salts Substances Youtube

Chemistry Ii Water And Organic Molecules
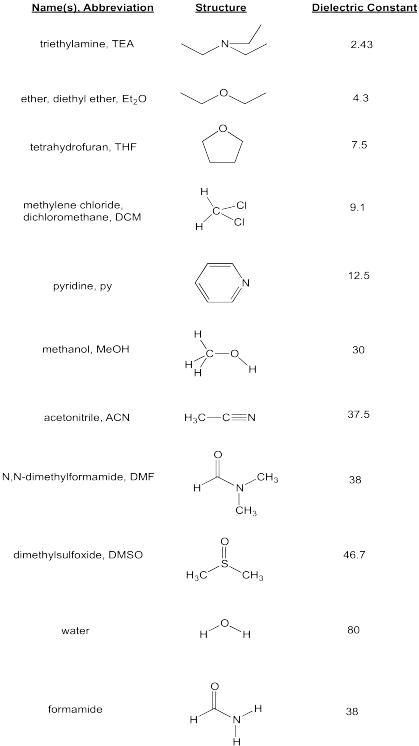
Solubility Factors When Choosing A Solvent News Announcements Cayman Chemical

Advanced Layering Over A Water Soluble Under Drawing Carrie L Lewis Artist Colored Pencil Tutorial Colored Pencil Techniques Pencil Drawing Tutorials
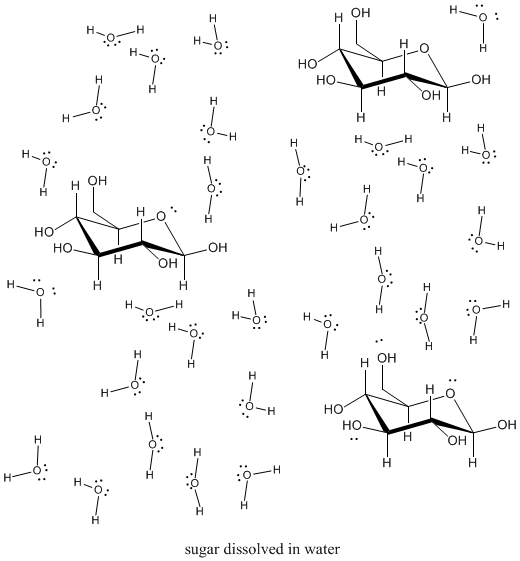
7 5 Aqueous Solutions And Solubility Compounds Dissolved In Water Chemistry Libretexts

Retinol Also Known As Vitamin A1 Is A Vitamin Found In Food And Used As A Dietary Supplement As A Supplemen Chemistry Cooking Whole Chicken Chemical Structure



Comments
Post a Comment